The StarPax Investor Deck
Financial model
Construction, over of a period of 7 years, of 25 Starpax Cancer Centers on a market potential of 580 in the USA after FDA approval.
Starpax Cancer Centers (SCC)
ONE STARPAX CANCER CENTER = 10 PolarTraks + 2 MRI + 1 CT Scan + Building + Equipment + Starpax Medical Staff
2 MRI
1 CT SCANNER
| after FDA Approvalyear | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Cares centers in construction | 1 | 2 | 4 | 8 | 10 | 12 | 12 |
| Care centers in start-up (50% occupied) | 0 | 1 | 2 | 4 | 8 | 10 | 12 |
| Care centers in full operation | 0 | 0 | 1 | 3 | 7 | 15 | 25 |
Forecast financial performance
25 Starpax Cancer Centers in operation, on a market potential of 580 in the USA.
EBITDA of $348 Million USD in Year 2 and $12 Billion in Year 7.
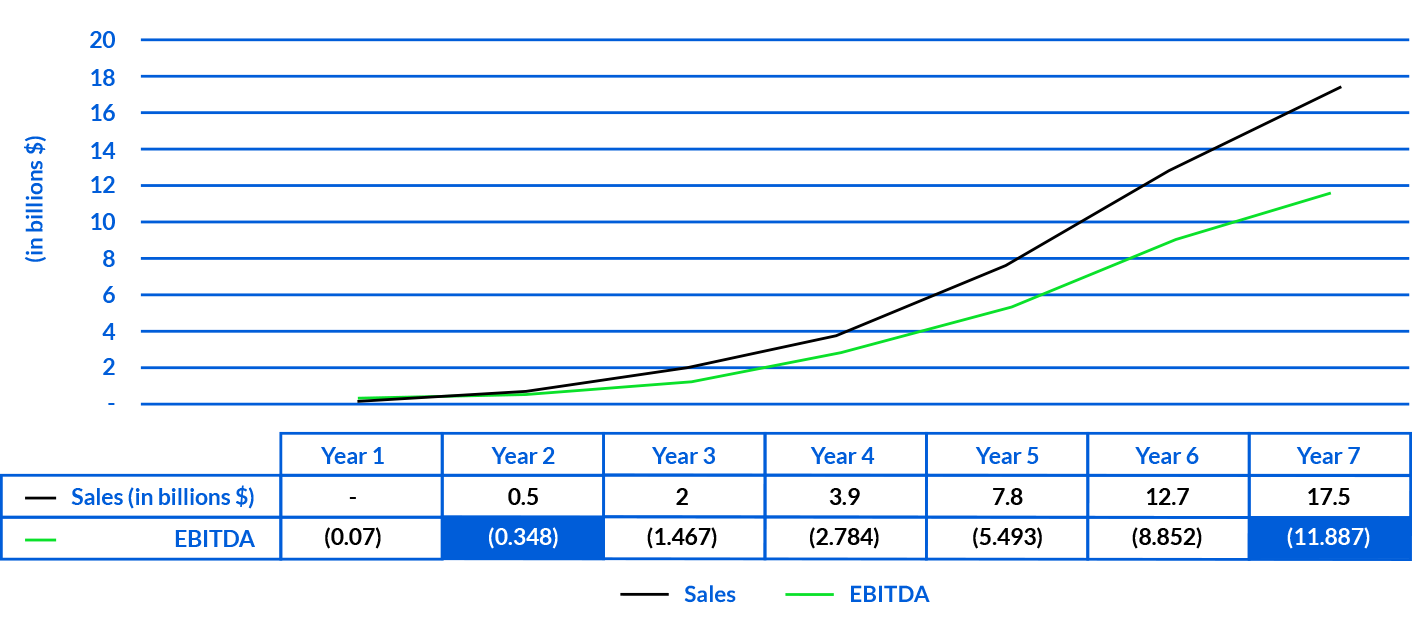
* These financial projections are based on assumptions that could be subject to revisions in the future and actual results may materially differ
Capitalization table
| Shareholder | #shares | % of Total |
| IPAX 2 | 53 750 860 | 71.19% |
| University (patents) | 7 500 000 | 9.93% |
| Institutional | 833 335 | 1.10% |
| Accredited Investors | 13 415 805 | 17.77% |
| Total | 75 500 000 |
Notes :
(1) Ipax 2 llp owns 71.19% of all Common Shares outstanding. Ipax 2 llp is owned 92.5% by MG Trust. Michael Gareau, founding-president, is a beneficiary of MG Trust. Michael Gareau is therefore the beneficial owner of 65.9% of the Company. Executive VP and COO is a beneficiary of a trust that owns 1.1% of Ipax 2 llp.
(2) CFO was granted 150,000 stock options. BoD was granted 630,000 stock options
The Starpax Timeline
A major critical problem to beat cancer
90%
of the volume of the tumor receives little or no drug at all
Source: Soltani & Chen (2012)
The Starpax Technology is Conceived to Distribute the Drug Throughout the Volume of the Tumor
This problem has persisted for more than a century
With systemic administration (injection in the blood system), anticancer drugs travel in the blood vessels, and their distribution throughout the volume of the tumor is restricted by the formation of hypoxic zones that result in the creation of chaotic, malfunctioning, or collapsed blood and lymphatic vessels and augment metastasis, which altogether further induces therapy resistance.
Think of an Amazon delivery truck arriving in a city hit by a magnitude 10 earthquake on a Richter scale. Roads and bridges are inaccessible, broken, or destroyed. No road means no delivery throughout the city. This is what happens to the delivery of drugs in a tumor.
solving such a problem takes more than a drug
Starpax is not an ordinary pharma. It has conceived a unique, groundbreaking technology harnessing the power of four different scientific disciplines:
- Microbiology
- Electromagnetic Engineering
- Biochemistry
- Artificial Intelligence
To address this critical problem that is insurmountable by relying on drugs or immunotherapy alone, we have created the Starpax Magnetodrones™ and the Starpax PolarTrak®, two patented components that must work with one another and conceived to solve what chemotherapy or immunotherapy have not solved for a century.
Our results
(preclinical studies analyzed by independent laboratories)
PRECLINICAL STUDIES ON HUMAN TUMORS GROWN IN ANIMALS
TESTED ON HCT 116 TUMOR (one of the most aggressive human tumors)
100%
remission rate*
0
meaningful side effects observed*
50x
more drugs in the tumor**
800x
fewer toxic molecules in the body**
(*) Based on Preclinical Studies
(**) Dose calculation according to clinical trials protocol to be used, compared to traditional chemotherapy
The Starpax Technology is not yet approved for commercial use. Clinical trials on humans are planned to begin in 2025.
Starpax Technology
The Artificial intelligence driven
PolarTrak
The PolarTrak is controlled by artificial intelligence that defines, from MRI imaging data, the exact shape and volume of the tumor in order to force the Magnetodrones to spread throughout the whole volume of the tumor while releasing the drug along their path.
Magnetic fields produced by the PolarTrak are safe for humans, as they are 2,000 times less powerful than those of an MRI.

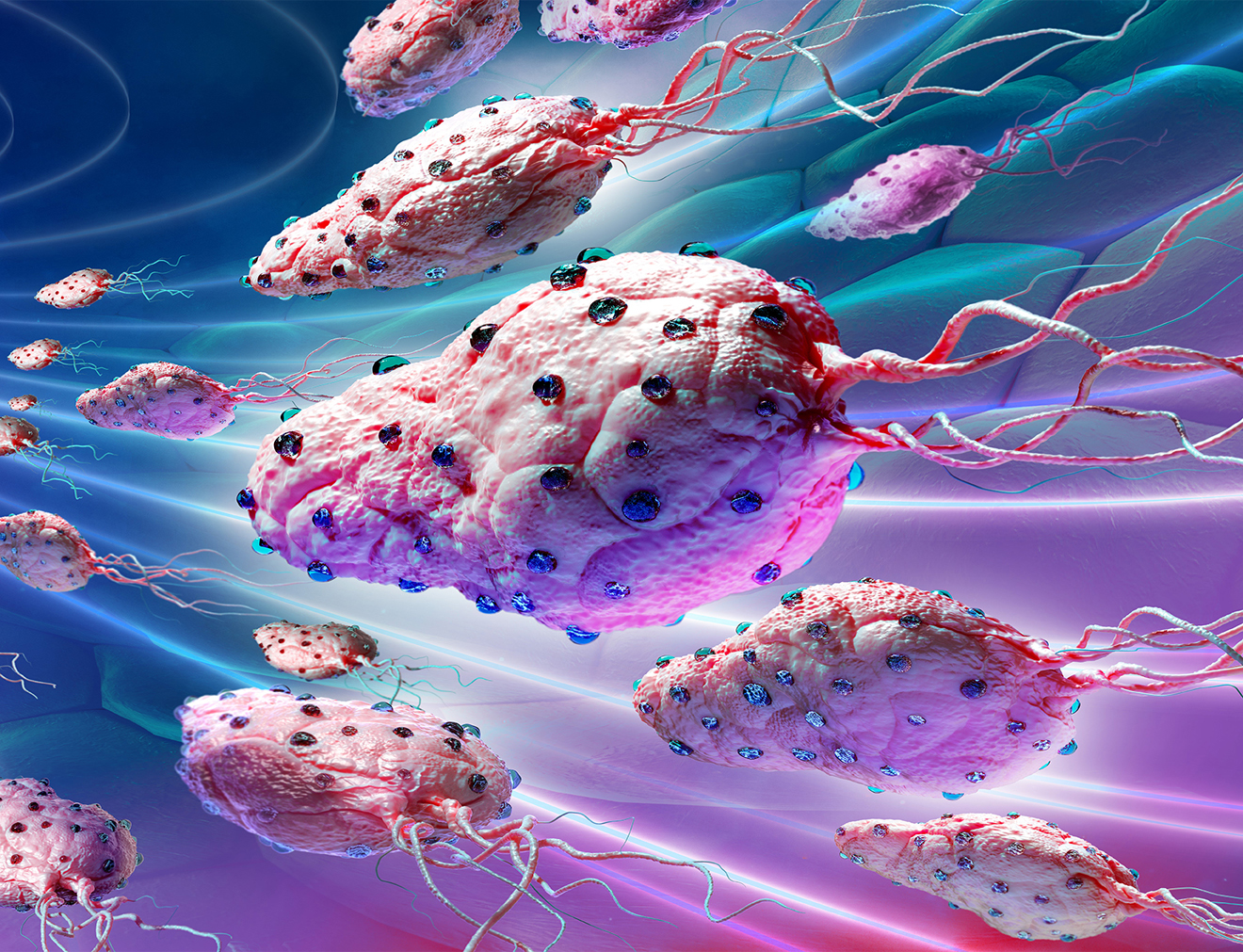
Self Propelled Non-Pathogenic Bacteria
Magnetodrones
Magnetodrones consist of unique proprietary self-propelled non-pathogenic bacteria (Bn1-S™) that are sensitive to magnetic fields and transport the drug attached to their surface. They can swim within interstitial spaces between tumor tissues without the need for blood vessels.
Magnetodrones are also aerotactic, meaning they naturally accumulate in the “low oxygen” hypoxic zones containing stem cells due to the level of oxygen in the hypoxic zones being the same as in their culture medium. They bring an anticancer molecule proven to work in hypoxic areas, where stem cells don’t split.
They are injected directly into the tumor, do not proliferate in the human body, and die within 60 minutes.
Summary of significant advantages
GROUNDBREAKING TECHNOLOGY WITH LIFE-CHANGING RESULTS
100%
remission rate in preclinical studies
800x
fewer toxic molecules in the body than traditional chemotherapy*
(*) Dose calculation according to clinical trials protocol to be used, compared to traditional chemotherapy
3
therapeutic benefits in one treatment
- Localized chemo inside the tumor to avoid side effects and kill cancer cells in the tumor
- Hypoxic zones penetration & therapy attacking stem cells in hypoxic zones
- Immunotherapy throughout the patient’s body, without having any drug circulating in the rest of the patient’s body.
50x
more drug in the tumor. Making it more effective than traditional chemotherapy while avoiding drug circulation in the bloodstream.*
(*) Dose calculation according to clinical trials protocol to be used, compared to traditional chemotherapy
Fighting cancer stem cells in hypoxic zones. Low-oxygen areas in which chemotherapy, radiation therapy, and immunotherapy have been proven ineffective.
Reducing the risk of toxicity and efficacy failure in clinical trials. By using anticancer molecules already in use in FDA-approved anticancer treatment for over 20 years in humans.
10
-minute injection directly into the tumor. Administered in an outpatient setting.
Board of directors

Michael GAREAU
Founding President and
Chairman

John HELOU
Retired President
PFIZER Canada

Lisa MATAR
Retired President
ELI LILLY Canada

André MONETTE
Retired President
JOHNSON & JOHNSON France

Dr. Jacques JOLIVET, M.D.
Oncologist
George V Silver Jubilee Award in Oncology

Pierre DOZOIS
Business Lawyer
Found Partner BCF LAW FIRM

Berthe LATREILLE
Retired COO
United Kingdom
Three therapeutic benefits in one treatment
Localized Chemotherapy Inside the Tumor*
The Magnetodrones™ are trapped inside the tumor with the drug attached to their surface.
The Magnetodrones™ cannot infiltrate the blood system because their size is bigger than the holes in capillaries.
Therefore, the Magnetodrones™ are restricted from exiting the tumor and circulating in the patient’s body to cause damage to organs or other side effects.
Hypoxic Zones Penetration & Therapy*
Hypoxic zones in a tumor are areas where oxygen is virtually absent. Hypoxic zones are where stem cells are located; they are a primary driver of metastatic spread.
When the Magnetodrones™ pass by a hypoxic zone, they stop “swimming” and accumulate inside the hypoxic zone due to the level of oxygen within the hypoxic zone being the same as in their culture medium. They release a specific drug proven to work in hypoxic areas where stem cells are located.
For decades, several studies have demonstrated that chemotherapy, radiotherapy, or immunotherapy are barely effective in hypoxic zones.
Immunotherapy*
A preclinical studiy has demonstrated that introducing the living Starpax Magnetodrones™ alone into a tumor, without transporting the drug, triggers the immune cells, and a substantial regression of the tumor was observed.
The Starpax treatment may cause a systemic immune response generating immune cells that could attack floating cancer cells in the rest of the patient’s body and remote metastases, without any Magnetodrones™ or anti-cancer drug circulating in the body.
*See disclosure page. The Starpax Technology is not yet approved for commercial use. Clinical trials on humans are planned to begin in 2025.
FAQ
Regulation A+
Q: What is a Regulation A+?
A: Reg A+ is a SEC regulation that allows a company to use crowdfunding to raise capital. As of March 15, 2021, Reg A+ rules allow companies to raise up to $75 million in a 12-month period. (https://www.bartonesq.com/news-article/reg-a-offerings-faqs/#what-is-a-reg-a-offering)
Q: Who can invest in Starpax Biopharma Inc. under the Regulation A+ offering?
A: Only US resident or citizen accredited, and non-accredited investors are allowed to invest in Starpax Biopharma Inc. under the Regulation A+ offering.
Q: Where will be my shares?
A: Using a similar procedure of investing at a stock exchange, your shares can be viewed under your name and personnal account that will be set up by the registrar and transfer agent KoreConX that is fully compliant with SEC regulations. Electronic certificate of your shares will be registered on the All-in-One electronic registrar platform KoreConX.
Q: Will I received a Share certificate?
A: There will be no physical share certificates. All electronic share certificates are registered under the name of the investor and can be accessed in the investor personal account on the All-In-One Platform KoreConX.
Q: Can I resell my shares?
A: Yes, you can make over-the-counter transfers using the All-In-One Platform KoreConX.
Q: Will Starpax Shares be on a public exchange?
A: Starpax intends to list on NASDAQ and/or TSX in a near future depending on market conditions. For the moment, the Company Shares are not currently listed on any exchange. However, the Shares will be transferable, in accordance with Federal and state securities laws, and Canadian laws. Investors are urged to consult their own legal advisors with respect to secondary trading of the Shares.
Q: Starpax has to comply to which securities laws?
A: An offering statement has been filed with the Securities and Exchange Commission (the “SEC”). Please refer to the Offering Statement : https://www.sec.gov/Archives/edgar/data/1960682/000196068223000003/partiiandiii.htm
Q : Is there a deadline to invest?
A:. The offering is live for a period of 12 months commencing on September 11, 2023 but Starpax reserves its right to terminate the process at any time.
Q : Is there any cost to investing?
A: Broker-dealer fees and commissions are paid by the issuer. The investor may have to pay for its customary bank fees, as well as its own advisory fees if required.
Q : Why do you require my social security number to invest?
A : We understand your concern regarding the security of your Social Security Number (“SSN”). This information is required by the SEC and FINRA to comply with the KYC (“Know-Your-Client”) and the AML (“Anti-Money Laundering”) rules. This information is validated by and made available only to the registered broker-dealer.
Starpax Technology
Q: Can Bn1-S bacteria transport other types of drugs?
A: Magnetodrones are envisioned to carry a wide array of drugs, including anti-cancer medications and various other drug categories. The development of a specific liposome for each drug will be necessary. Potentially, Bn1-S could serve as a carrier for diverse therapeutics, encompassing immune checkpoint inhibitors, contrast agents, antibiotics, and more. (For additional information: https://starpaxbiopharma.com/pipeline/)
Q: Will Magnetodrones transporting other drugs require individual FDA approvals?
A: It is anticipated that a distinct approval will be mandated for every novel pairing of a therapeutic agent with the Magnetodrones. This adheres to the standard FDA requirement, where each new formulation or combination necessitates specific approval within the realm of medical technologies.
Q: Beyond the initial six types of cancers, which other cancer types are being considered?
A: Starpax Therapy is designed to address solid tumors that represent 90% of cancer and 89% of deaths. In this context, Starpax aspires to extend the application of its technology to encompass all potential indications.. We envision a promising future for Starpax’s innovative modality, viewing it as a transformative industry breakthrough with significant positive societal impacts.
Q: Can the technology be applied to children?
A: In technical terms, yes, it is feasible. However, as is customary with many medical technologies, the technology initially requires approval for use in adults. After obtaining approval for adult use, Starpax intends to proceed with further clinical development to seek approval for application in children.
Q: Is it possible to treat brain cancers?
A: Indeed, the Starpax Platform is designed with the potential to address cranial and brain cancers. However, this necessitates further interventional assessments and development to confirm the appropriate procedures. Starpax is committed to commencing this work after the beginning of the forthcoming clinical trials.
Q: Can Starpax Technology become a first-line treatment?
A: In stark contrast to the majority of new cancer treatments in development that may never attain first-line status, Starpax firmly believes that its technology possesses the attributes necessary to become a first-line treatment. This conviction is rooted in several key factors:
- Starpax technology is designed to circumvent the toxicity associated with systemic treatments by containing the drug within the tumor with the magnetic vectors of the PolarTrak.
- It offers a non-invasive approach: simple intratumoral 10-minute injection of the Magnetodrones being done in an outpatient facility.
- The potential exists for preserving tissue and organ functions, mitigating risks associated with radiation therapy and surgical interventions.
- Starpax is anticipated to provide superior efficacy by delivering the drug throughout the tumor volume at significantly higher doses than systemic treatments.
- It specifically targets the often hard-to-reach hypoxic zones, which are responsible for metastasis and recurrence.
- Additionally, it acts as an immune trigger, further enhancing its therapeutic potential.
(For additional information: https://starpaxbiopharma.com/technology/#technology_sec_2)
Q: Can I volunteer for the clinical trials?
A: Patient recruitment for the clinical trials can only commence once authorization is granted by regulatory authorities. Unfortunately, we are not allowed to maintain a waiting list, confirm trials exact commencement date, or provide information on patient eligibility criteria at this time. We recommend consulting with your oncologist to explore currently available treatment options and ongoing clinical studies that are actively enrolling patients. You can access a specialized website for this purpose at https://clinicaltrials.gov/ct2/home. Each clinical study has distinct inclusion criteria, so it is essential to have a discussion with your oncologist to determine whether a specific study might be a suitable option for you. As for Starpax, announcements and communications will be disseminated to the oncologist community in the future. In the meantime, it is crucial not to delay consulting with your oncologist regarding your treatment options.

